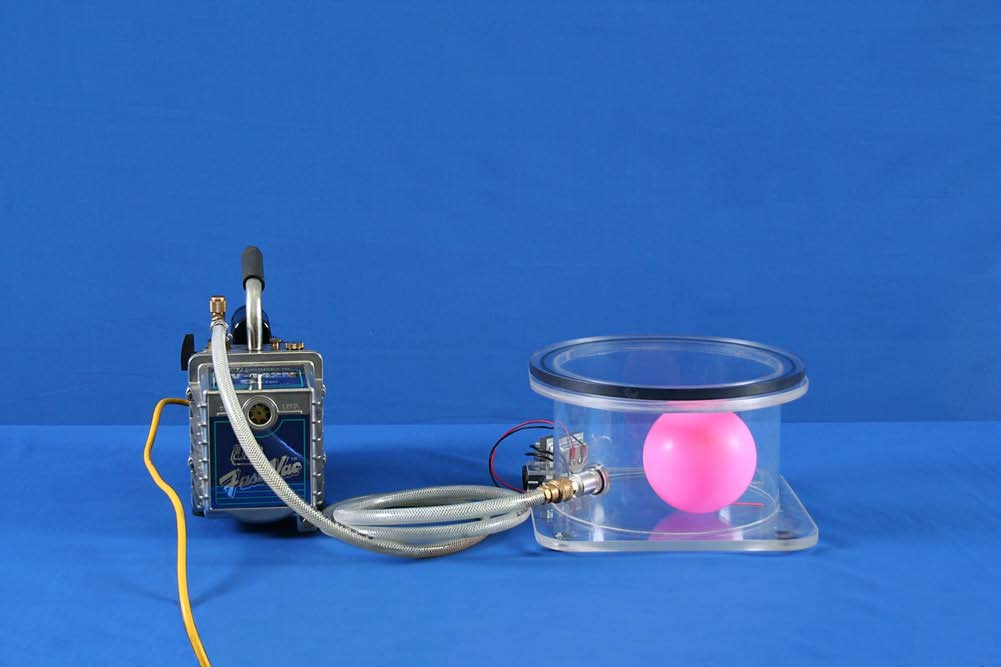


Concept
Demonstrates Boyle's Law which states that, “For a fixed amount of gas kept at a fixed temperature, $P$ and $V$ are inversely proportional (while one increases, the other decreases).”
Procedure
- Partially inflate the balloon and place it in the vacuum chamber (see picture).
- Cover the vacuum chamber with the lid.
- Connect the vacuum pump hose to the vacuum chamber 2.
- Plug the vacuum pump into a power outlet.
- Turn on the vacuum pump using the switch (1) at the back of pump and watch the balloon grow.
- Turn off the pump and close the vacuum valve (2) to prevent air leaking.
- Unscrew one of the top brass caps (3) in order to release the air in the chamber.
- When the chamber has been filled with air (i.e., no more hissing noise), disconnect the pump 3 from the vacuum chamber.
- Remove the lid and take out the balloon.
Equipment
- Transparent vacuum chamber
- Vacuum pump with hose
- Medium (or large) balloon