
Concept
The direct proportionality between pressure and temperature is clearly shown here for a fixed volume of gas.
Procedure
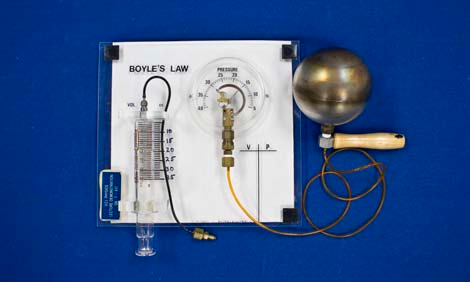
- Verify that the constant volume bulb is securely connected to the pressure gauge.
- Note the pressure reading at room temperature.
- Fill the beaker about 2/3 full with liquid nitrogen and place the bulb in the liquid nitrogen bath.
- Watch the pressure in the bulb fall.
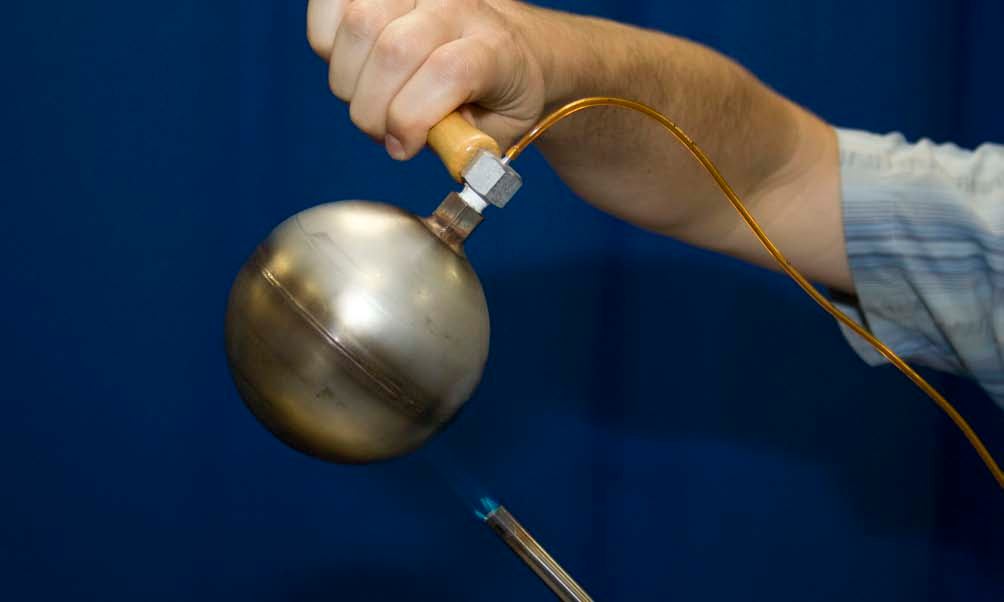
- Light the propane torch and carefully hold the bulb over the flame.
- Watch the pressure in the bulb rise.
Equipment
- 2000 mL Pyrex Beaker
- Liquid Nitrogen
- Propane Torch
- Boyle’s Law Pressure Gauge
- Constant Volume Bulb
- Metal Tray
- Overhead Projector or Document Camera (not pictured)
- Large Display Thermometer (not pictured, available upon request)