Volume 77, Number 48
CENEAR 77 48 pp.
ISSN 0009-2347
Scanning instrument manipulates molecules, makes bonds, images products, and, now, measures molecular vibrations
You'd hardly think of a scanning tunneling microscope (STM) as a Swiss Army knife. But from the way researchers at Cornell University use their STM to manipulate and synthesize molecules, image reactants and products, and measure vibrational spectra, thoughts of the handy all-purpose tool come to mind.
Cornell physics professor Wilson Ho and graduate student Hyojune Lee have just demonstrated the multifunctional quality of their homemade scanning probe instrument by constructing metal carbonyls in a stepwise fashion and analyzing the molecules' bonds one at a time [Science, 286, 1719 (1999)].

Standing in their
STM laboratory, Ho (left) displays a metal evaporator and Lee holds a scanning
assembly.
The Cornell study may lead to better understanding and control of surface chemistry at the atomic or molecular level. Such information is used to devise models and verify mechanisms in heterogeneous catalysis and molecular epitaxy and may aid development of molecular electronics and sensors.
"This is a beautiful paper," remarks John H. Weaver, STM expert and professor of materials science and engineering at the University of Minnesota, Minneapolis. "It should capture the imagination of anyone who has ever wanted to visualize an atom and the construction of molecules." Weaver adds that the methods developed by the Cornell physicists are "very elegant and should be extendable to other systems."
Starting with a pristine silver specimen, Ho and Lee prepare the platform on which chemical reactions will be carried out by delivering to the silver surface trace quantities of iron atoms and carbon monoxide molecules.
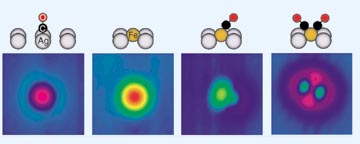
STM makes and
probes bonds one at a time
With atomic-level dexterity, Cornell physicists pick up a carbon monoxide molecule from a silver surface (left) and deliver it to a nearby iron atom (center left), making Fe(CO) (center right). Repeating the procedure gives Fe(CO)2 (right). Analysis of STM images (color coded to reveal topography) indicates that C-O bonds are tilted relative to the silver surface.
Displaying angstrom-scale deftness, the Cornell researchers then pick up a single CO molecule and position it over an isolated iron atom. By adjusting properties of the tiny electrical current flowing to the STM's tip, the group relaxes its grip on the molecule and releases it to the iron atom, encouraging the species to form Fe(CO). Repeating the maneuver, the physicists convert the metal carbonyl to the dicarbonyl, Fe(CO)2.
To get a close look at the chemistry proceeding on the silver surface, Ho and Lee interrogate their specimen via two methods: STM imaging and STM-based vibrational spectroscopy. Images reveal changes in the appearance of the atomic landscape as bonds are formed and molecules are constructed. Vibrational measurements identify and characterize reactants and products quantitatively.
"STMs are not inherently chemically sensitive tools," Ho points out. "Doing vibrational spectroscopy with an STM has been a Holy Grail of sorts for quite some time." A procedure that combines vibrational measurements with an STM's atomic-scale spatial resolution would enable scientists to study vibrational spectra of single molecules, he adds.
Last year, Ho's group demonstrated that an STM, in fact, can be used for single-molecule vibrational spectroscopy and microscopy [Science,280, 1732 (1998)]. The technique, known as inelastic electron tunneling spectroscopy, relies on small, sharp changes in an electrical current that flows between an STM tip and an adsorbed molecule. Variations in current depend on the energy of tunneling electrons. A signal is observed when the energy of the electrons matches the energy of a molecular vibration.
Applying the method to the iron carbonyl system, the Cornell physicists show that the procedure is sensitive enough to distinguish between isotopes. The C-O stretch in Fe(12C16O), for example, occurs at 236 mV, whereas in Fe(13C18O) that vibrational band is shifted to 224 mV. The procedure also differentiates Fe(CO) from Fe(CO)2 and discriminates among isotopes of Fe(CO)2.
Ho acknowledges that recording vibrational spectra with an STM is challenging
experimentally. Part of the researchers' success, he says, lies in the
versatile STM software that they developed and in the measures taken to
ensure the mechanical and thermal stability of their instrument. But it's
also critical to apply the technique to appropriate chemical systems. The
researchers now plan to turn their attention to bonding of single molecules
to other isolated atoms and aggregates of atoms and to devising additional
manipulation techniques to investigate bimolecular reactions.
Chemical & Engineering News
Copyright © 1999 American Chemical Society