 |
|
|
|
 |
|
|
 |

|
 |
 |
 |
 |
 |
 |
 |
Single Molecule Sensing |
Because of their quasi one-dimensional electronic structure and low carrier concentration,
SWNT FETs demonstrate extreme electronic sensitivity to environmental perturbations.
This sensitivity enables the electrical detection of minute chemical changes. Our
strategy concentrates this sensitivity to a single position by either (1) introducing a
covalent defect or (2) noncovalently attaching a single receptor molecule.
With the sensitivity amplified at a single position, devices can monitor the binding and
unbinding of single molecules.
In the case of covalent defects, enhanced scattering by a defect site can overwhelmingly
dominate the electrical characteristics of the whole device. Consequently, any chemical
degrees of freedom of the defect are transduced into an electrical signal. This has proven
particularly useful for looking at chemically-active defect sites, such as -COOH defects
exposed to EDC molecules. Some of our research examines the physics of these sites using
scanning probe microscopy.
The noncovalent attachment strategy is more general and allows us to study the degrees of freedom
of any attached molecule. For example, we can monitor the conformational changes of a protein as
it catalyzes chemical reactions. This technique has now used to study a number of
enzymes with single molecule precision.
In order to design, build, and further exploit these sensing capabilities, a large part of our
research is focused on understanding the mechanisms at work. With proteins, we find that signal
transduction is governed by the scattering barrier induced by protein attachment. Movements of
the protein, and specifically of its charged amino acids, can electrostatically gate this
scattering barrier and modulate the SWNT conductance. We have tailored the strength of this effect
by site-directed mutagenesis, in which selected amino acids can be made charged or neutral. By
considering the motions and charges of any particular enzyme, we can design an orientation for
measuring single molecule dynamics without any fluorophore labels.
|
 |
|
|
 |
Publications |
Dissecting Single-Molecule Signal Transduction in Carbon Nanotube Circuits with Protein Engineering
Y. Choi, T.J. Olsen, P.C. Sims, I.S. Moody, B.L. Corso, M.N. Dang, G.A. Weiss & P.G. Collins,
Nano Lett. 13, 625-31 (2013).
Single-Molecule Lysozyme Dynamics Monitored by an Electronic Circuit
Y. Choi, I.S. Moody, P.C. Sims, S.R. Hunt, B.L. Corso, G.A. Weiss & P.G. Collins
Science 335, 319-24 (2012).
Scanning Gate Spectroscopy and Its Application to Carbon Nanotube Defects
S.R. Hunt, D. Wan, V.R. Khalap, B.L. Corso & P.G. Collins,
Nano Lett. 11, 1055-60 (2011).
Hydrogen Sensing and Sensitivity of Palladium-Decorated Single-Walled Carbon Nanotubes with Defects
V.R. Khalap, T. Sheps, A.A. Kane & P.G. Collins,
Nano Lett. 10, 896-901 (2010).
Monitoring Single Molecule Reactivity On a Carbon Nanotube
B.R. Goldsmith, J.G. Coroneus, A.A. Kane, G.A. Weiss, & P.G. Collins,
Nano Lett. 8, 189 (2008).
Conductance-Controlled Point Functionalization of Single-Walled Carbon Nanotubes
B.R. Goldsmith, J.G. Coroneus, V.R. Khalap, A.A. Kane, G.A. Weiss & P.G. Collins,
Science 315, 77 (2007).
|
 |
Acknowledgements |
| This research is financially supported by the NSF and the NIH. |


 |
|
|
|
 |
|
|
|

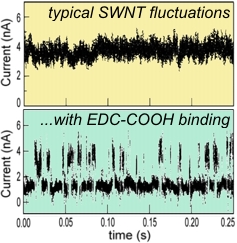
Covalent binding an unbinding of EDC to a -COOH interactions between a protein's charged amino
acids and a SWNT transistor.

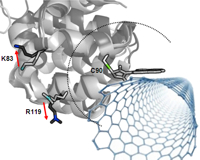
Electrostatic interactions between a protein's charged amino acids and a SWNT transistor.

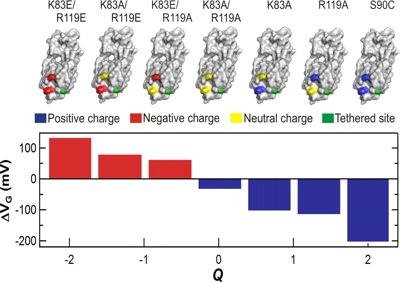
Strength and sign of interactions are proportional to the protein's moving charges.
|
 |


