Abstract
Introduction
Experimental Setup
Data
Discussion
Results
References
|
 |
Discussion
 Pandit
and Fisher [10] pointed out that the stability of various surface phases
depends critically on the relative size of the surface tensions slv,
ssw, ssl,
slw, and svw
(where l=liquid, s=solid, v=vapor and w=wall), and that a large number
of surface phase transitions are possible in the vicinity of the triple
point. Pettersen Lysek, and Goodstein [14] have constructed a simple phenomenological
model in which the film is regarded as a slab or slabs of bulk phase with
the surface tension, van der Waals potentials and other effects treated
as perturbations. We have used this type of model to interpret our data.
The basis of the method is to write the grand free energy W(m,T;z),
for a film of arbitrary thickness z and minimize W
with respect to z. Near the triple point, there are three free energies
Wl, Ws,
and Wlay, for the liquid, solid and
layered film which need to be considered; the stable phase is of course
the one with the lowest value of W. For example,
the free energy of the layered phase for arbitrary values of the thickness
of the liquid and solid slabs zl and zs is: Pandit
and Fisher [10] pointed out that the stability of various surface phases
depends critically on the relative size of the surface tensions slv,
ssw, ssl,
slw, and svw
(where l=liquid, s=solid, v=vapor and w=wall), and that a large number
of surface phase transitions are possible in the vicinity of the triple
point. Pettersen Lysek, and Goodstein [14] have constructed a simple phenomenological
model in which the film is regarded as a slab or slabs of bulk phase with
the surface tension, van der Waals potentials and other effects treated
as perturbations. We have used this type of model to interpret our data.
The basis of the method is to write the grand free energy W(m,T;z),
for a film of arbitrary thickness z and minimize W
with respect to z. Near the triple point, there are three free energies
Wl, Ws,
and Wlay, for the liquid, solid and
layered film which need to be considered; the stable phase is of course
the one with the lowest value of W. For example,
the free energy of the layered phase for arbitrary values of the thickness
of the liquid and solid slabs zl and zs is:
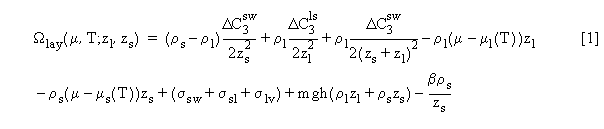
where ri are the densities, mi(T)
are the bulk chemical potentials, and DC3ijare
differences of van der Waals C3 coefficients for the various
bulk phases. 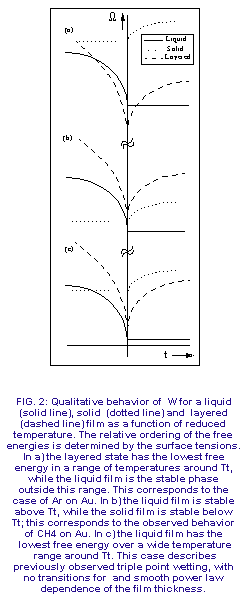 The
terms proportional to m-mi(T) express
the cost of forming a phase away from bulk coexistence. The term containing
the surface tensions sij accounts
for the energy required to form solid-wall, solid-liquid and liquid vapor
interfaces. The term proportional to mgh accounts for the gravitational
potential energy of the film, while the last term proportional to b
accounts for the strain energy in the solid part of the film [1, 2]. Similar
terms appear in Ws and Wl.
See references [14,15] for further details. The
terms proportional to m-mi(T) express
the cost of forming a phase away from bulk coexistence. The term containing
the surface tensions sij accounts
for the energy required to form solid-wall, solid-liquid and liquid vapor
interfaces. The term proportional to mgh accounts for the gravitational
potential energy of the film, while the last term proportional to b
accounts for the strain energy in the solid part of the film [1, 2]. Similar
terms appear in Ws and Wl.
See references [14,15] for further details.
 Equation
(1) and its analogues for Ws and
Wl can be numerically minimized with
respect to the zi. The equilibrium W,
the surface phase, and the film thicknesses are then determined by the
lowest lying branch at each temperature. The relative positions of the
three branches are determined by the magnitudes of the surface tensions.
Figure 3 shows three different orderings of the free energies with m=the
bulk coexistence value. The orderings correspond to different sequences
of surface phase transitions near Tt. The qualitative behavior
of each free energy branch is independent of the values of the parameters.
The single phase branches have essentially constant values of W
as long as the corresponding bulk phase is stable, with a cusp-like rise
at the triple point where the bulk phase becomes metastable. Wlay
is V-shaped with a sharp minimum at Tt. Figure 3a corresponds
to the Ar surface phase diagram obtained from the data in Figure 1 with
a high and low temperature liquid phase separated by a layered phase near
Tt. Figure 3b shows a direct transition from liquid to solid
at a temperature very close to Tt, which we believe is the case
for CH4. Figure 3c illustrates the triple point wetting scenario
inferred from all previous experiments in which liquid films coexist with
bulk solid over a wide range of temperature. Equation
(1) and its analogues for Ws and
Wl can be numerically minimized with
respect to the zi. The equilibrium W,
the surface phase, and the film thicknesses are then determined by the
lowest lying branch at each temperature. The relative positions of the
three branches are determined by the magnitudes of the surface tensions.
Figure 3 shows three different orderings of the free energies with m=the
bulk coexistence value. The orderings correspond to different sequences
of surface phase transitions near Tt. The qualitative behavior
of each free energy branch is independent of the values of the parameters.
The single phase branches have essentially constant values of W
as long as the corresponding bulk phase is stable, with a cusp-like rise
at the triple point where the bulk phase becomes metastable. Wlay
is V-shaped with a sharp minimum at Tt. Figure 3a corresponds
to the Ar surface phase diagram obtained from the data in Figure 1 with
a high and low temperature liquid phase separated by a layered phase near
Tt. Figure 3b shows a direct transition from liquid to solid
at a temperature very close to Tt, which we believe is the case
for CH4. Figure 3c illustrates the triple point wetting scenario
inferred from all previous experiments in which liquid films coexist with
bulk solid over a wide range of temperature.
|
|

