 |
Latest News
MEMS, Liquid Lenses and Pulsed-laser Deposition research added.
|
|
|
|
| Dr. Taborek
|
|
|
| Publications |
|
|
| Students |
|
|
| Postdocs |
|
|
|
|
|
Alumni |
|
|
| Current Research |
|
|
| Contact Us |
|
|
Handbook  |
|
|
|
|
|
| UCI
|
|
|
| Physical Sciences |
|
|
| Physics Dept. |
|
|
|
|
|
|
 |
| |
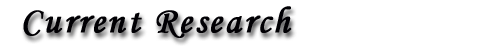
|
|
- Cryogenic characterization of MEMS
top
- Micro-electro-mechanical systems (MEMS) are mechanical devices built on silicon
wafers at the micron scale. These devices have been in existence for almost as long
as the integrated circuits which are now found in computers. However, due to the
complexity and difficulty in design and fabrication, as well as the strong
sensitivity (sometimes oversensitivity) of the devices, they have not become as widely
used. They are currently employed in a number of applications as pressure sensors
and accelerometers, to name a few. In order for more complex MEMS devices to become
widely used, the behavior of these devices must be studied. We are studying a MEMS
resonator in air and vacuum from room temperature to 6K. Specifically, we intend to
study how wear, stiction, and resonance change while changing pressure and temperature.
There is clearly a strong difference between the resonance curves for the MEMS device
in air and under vacuum at room temperature. The quality factor, Q, of the device
changes from 5 in air to 40,000 at 5x10-6 Torr.
- Pulsed Laser Deposition
top
- Preparing clean, uniform surfaces is difficult at cryogenic temperatures.
In the past we have utilized thermal evaporation where a small container of Cesium
or other metal is heated to 400K or above in a 4K environment. The metal is
evaporated onto a quartz crystal microbalance (QCM). This method has two main
difficulties; it becomes difficult to control how much material is placed on the QCM
because of heating effects and this method adds a lot of heat to our cold system.
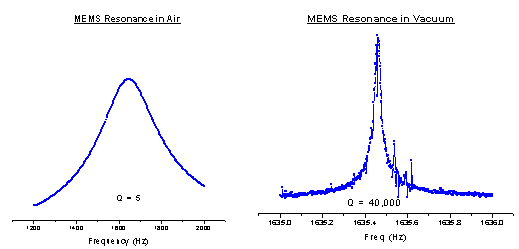
To solve these problems we have developed another deposition method called Pulsed
Laser Deposition (PLD). In this setup we utilize a Nd:YAG laser set to its second
harmonic, 532nm light. This laser light is pulsed and has a pulse width of 10 ns.
The light is brought into the cell and strikes a cold target. The local target area
is heated up to thousands of degrees and material flies off the surface. A QCM is
placed perpendicular to the surface of the target, as seen below, and the material
is deposited onto the QCM.
This method has the benefits of low heating and a great deal of control. We have
sublayer deposition control with this setup.
- Liquid Lenses
top
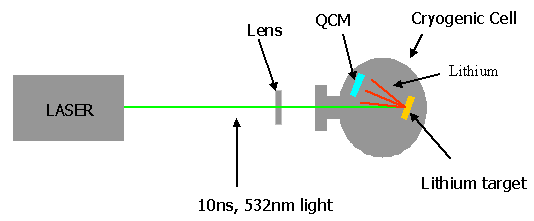
- Pinch-off in conventional 3D droplets and bubbles have been of considerable
recent interest. In 3D, pinch-off is driven by surface tension, and various flow
regimes have been observed which depend on the viscosity and density ratios of the
interior and exterior fluids. In contrast, 2D line tension alone can not drive a
film to break apart. Liquid lenses provide an interesting intermediate case in
which surface tensions are small and the geometry approaches 2 dimensions. We have
developed an experimental system to reproducibly observe pinch-off in hydrocarbon
liquid lenses. The shape of the pinch region is qualitatively different than that
in 3D, and involves a hierarchy of multiple spontaneous singularities and
satellite lenses. High-speed video will be shown which illustrates these phenomena.
Video: Liquid decane pinch-off on the surface of water
 See the video See the video
- Wetting on Weak and Intermediate Strength Substrates
top

- What is wetting? If two coexisting phases, A and B, are in a container,
the walls of the container will prefer either A or B. The preferred
phase is said to wet the wall. The preference is quantitatively determined
by the size and sign of the surface free energies in the problem:
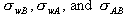
If 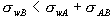 , then B wets the wall. , then B wets the wall.
The surface free energies depend on temperature and other variables, and the
inequality can be reversed as these variables are changed. In other words,
as the temperature is varied, the system can change its mind about which
phase it prefers. This is a wetting transition.
Although the word "wetting" implies liquids, the physics is very general
and applies to systems as varied as binary liquid mixtures, magnetic phases,
and superconductors. The main idea is that even when bulk phases have exactly
the same free energy and coexist, the surface breaks the symmetry and the
phases and phase transitions that occur on the surface can be very different
than in the bulk. These differences give rise to phenomena such as prewetting,
and affect processes such as droplet formation, contact angles & contact
angle hysteresis, nucleation, and flow which we investigate in our lab.
- Weak Substrates
top
- As noted above, wetting is the competition of phases for access to the wall.
The competition is interesting when it is closely matched. One way to
characterize the competition is interaction strengths. For example, if the
interaction between particles in a liquid is stronger than the interaction
between the liquid molecules and the wall, the liquid does not wet, and beads
up on the surface in the form of a droplet with a finite contact angle.
Liquid helium is a particularly weakly-interacting system. Since the
interaction of helium with almost anything else is stronger than the
helium-helium interaction, liquid helium was thought to wet everything. It
was therefore surprising when Cheng, Cole, Saam, and Treiner predicted that
helium would not wet cesium. The basic reason for this is that electrons are
very weakly bound to cesium, so an electron cloud extends unusually far from
a cesium surface; this cloud prevents the helium from getting very close to
the surface to take advantage of the van der Waals well.
- Cesium Deposition
top
- Cesium is so reactive that it burns spontaneously in air. Our lab has developed
the technology to fabricate clean, high quality cesium surfaces and has used
them for a number of novel experiments with helium. Our method of making cesium
films is to evaporate the elemental metal from a glass ampule. Cesium evaporates
nicely at about 50ºC. Cleanliness is ensured by keeping the rest of the
apparatus at 4 K.
- Prewetting transitions
top
- One method of monitoring the state of the surface is to use a quartz crystal
microbalance (QCM). We used QCMs coated with about 100 layers of cesium to
investigate the surface phase diagram of 4He on Cs. The system shows a wetting
transition at about 2 K and is the first experimental system to show a prewetting
line. Prewetting is a first-order phase transition that separates thick and thin
films, and is roughly analagous to a surface liquid-vapor transition.
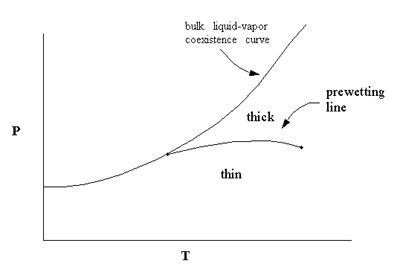
We have used this type of system to investigate wetting transitions in 3He,
and in 4He-3He mixtures.
- Superfluidity
top
- Another focus of our interest is in the relationship between wetting and
superfluidity. Superfluidity in thin helium films is a field which has been
intensely studied. It is the classical example of a Kosterlitz-Thouless phase
transition. It is particularly interesting on weak substrates because the film
has 4 choices: It can be super or normal, thin or thick. Bulk cesium is so weak
that liquid helium forms a superfluid film on it only very near the bulk lambda
point. We have made intermediate strength substrates by using extremely thin
cesium films or by using lighter alkali metals such as rubidium, potassium,
and sodium.
On both rubidium and thin cesium, we find that the so-called universal signatures
of the Kosterlitz-Thouless transition are not observed.
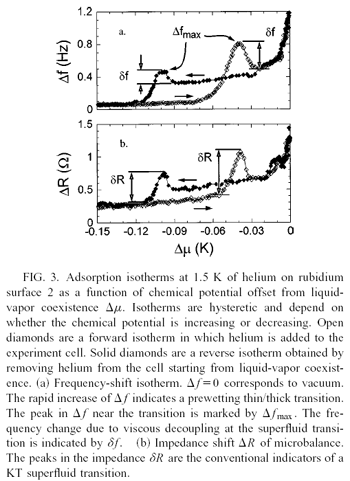
One reason for this non-universal behavior may be that localized solid-like layer
of helium (Bose glass) at the interface with a conventional substrate is absent
for intermediate strength substrates. In other words, on conventional substrate
materials, superfluids interact with the solid wall through a solid He layer,
while in weak substrates, the superfluid is in direct contact with the wall.
Mechanical oscillators in general and QCMs in particular have been the tool of
choice to study superfluid onset in thin films. The signature of superfluidity
is an apparent decoupling of mass when the superfluid fraction is no longer
viscously clamped to the motion of the substrate. On a weak substrate, however,
an apparent decoupling of mass can be due to either superfluid onset or
prewetting, and QCMs can not distinguish between the two possibilities, since
they only measure the normal fraction. To disentangle the effects of wetting and
superfluidity, we need a probe of the total (super + normal) coverage.
- Ellipsometry
top
- After assessing several possible techniques, we have decided on an ellipsometric
detection scheme. Briefly, an ellipsometer measures the rotation in the
polarization of light due to the adsorption of a thin film. More details. This
project is technically challenging because the small optical index of helium
(n = 1.03) produces very small polarization changes, and these can easily be
swamped by temperature dependent birefringence in the many windows that are
required in an optical cryostat. We have solved these problems and made
ellipsometric measurements of helium films with sub-monolayer resolution at 1.5 K.
Ellipsometric Study of Superfluid Onset in Thin Liquid 4He Films
- Superfluid Drop Dynamics
top
- Pinch-off top
When a piece of fluid separates into two droplets, there is a final instant
at which the new droplet is born. The fluid flows that produce the separation
are governed by highly nonlinear differential equations that have so-called
spontaneous singularities. The flows are a competition between surface
tension forces, which try to squeeze the fluid into two, and intertia and
viscosity, which resists this.

A superfluid droplet pinch-off: Compare this image to this one, taken a moment earlier.
This competition leads to universal icicle-like
shapes in the fluid flow which are very beautiful. We have investigated the
pinch-off process using superfluid helium to look for effects of viscosity and
possible unusual superfluid phenomena. We have studied superfluid pinch-off
using 5µs exposure flash photography and 1000 frame-per-second video.
 See the video See the video
Superfluid Drops: Dynamics of Pinch-Off and Sliding Motion
- Room Temperature Drop Dynamics
top
- Pinch-off top
We are also investigating pinch-off in droplets and bubbles at room temperature. We are
searching for a similar categorization of pinch-off behavior based on the viscosities
and densities of the inner and outer fluids. Interesting phenomena have been observed
while studying these regimes.
The videos below were made in a horizontal orientation. Refer to the figure below.
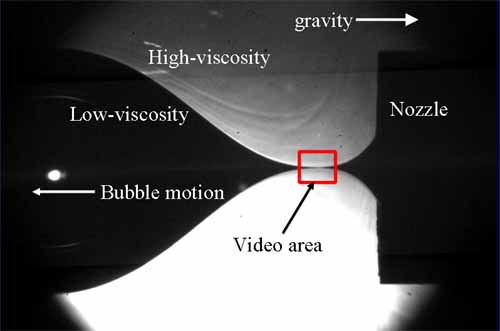
Video 1: Air bubbling through glycerin. The regime where the viscosity of the inner
fluid (air) is about 100,000 times smaller than the outer fluid.

 See the video See the video
Video 2: Water bubbling through heavy syrup. The regime where the viscosity of the inner
fluid (water) is about 13,000 times smaller than the outer fluid.
 See the video See the video
Video 3: Air bubbling through water. The regime where the viscosity of the inner
fluid (air) is about 1,000 times smaller than the outer fluid.

 See the video See the video
- Sliding droplets
top
- This video shows superfluid Helium-4 at 1.5K dripping off the capillary and landing
on a cesiated plate inclined at 21.5º from the horizontal. This angle is sufficiently
steep so that pinning forces are immediately overcome and the drop begins to slide
downhill. Remarkably, this measured acceleration amounts to approximately 20% of
the predicted gravitational acceleration.
 See the video See the video
 How did you tilt the cryostat? How did you tilt the cryostat?
- Dynamic friction in flow of droplets down an inclined plane
top
- The discovery of weak substrates which are not wet by helium opens up new
questions in superfluid dynamics. Conventional superfluid dynamics had no need
to consider the motion of a superfluid boundary, because the superfluid
immediately spread to wet all surfaces. On cesium substrates, drops of
superfluid can be formed which have an edge where superfluidity stops. The first
observation of these drops was reported in Superfluid drops on a solid surface.
The drops of superfluid behave in a counter-intuitive fashion: although the fluid
has no viscosity, the drops are very "sticky." When superfluid is poured onto a
cesiated inclined plane, it does not simply roll downhill. Rather, it will
ball-up and resist flow until a critical mass is reached. Apparently the contact
line is strongly pinned. This is surprising because the surfaces are formed in
ultra-high vacuum, are mirror-like, and show sharp steps in the prewetting
transition which can be observed on the same surfaces. We have measured the
contact line hysteresis for 4He on Cs.

There are strong pinning forces which prevent a drop from starting to move, but
what happens when it finally does move? Is there an effective frictional force
between the fluid and the plate? We have recently investigated this issue using
high-speed video of flowing drops. We find that superfluid drops have an almost
constant acceleration, but it is about 1/5 of the free-fall value of 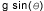 . .
- Classical fluid drop and contact line dynamics
top
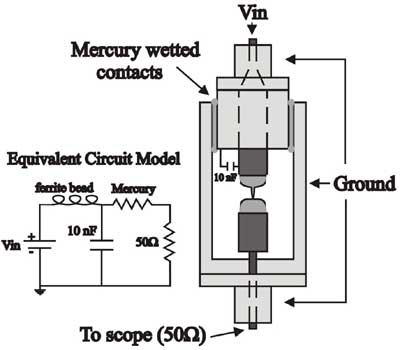
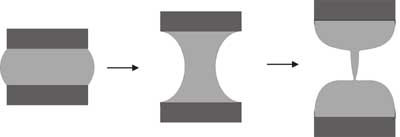
- Pinch-off in mercury top
Most experiments to investigate pinch-off have used optical images to look at the
minimum neck diameter. We have used this technique to look at superfluid pinch-
off. Optical techniques can follow the power law singularity predicted by fluid
mechanics only down to optical wavelengths, but they should continue to much
smaller length scales. We have developed an electrical technique based on
measuring the resistance of a pinching column of liquid mercury with a high-speed
digital oscilloscope. Using this technique, we can follow the dynamics of pinch-
off to within a few nanoseconds of the singularity.
- Dissipation in oscillating drops
top
- Our experience with dissipation of superfluid drops suggests that contact line
motion contributes to dissipation in an important but incompletely understood way.
Even for a classical liquid, the dissipation of a moving drop cannot be computed
directly from the Navier-Stokes equations. Attempts to do this run into non-
convergent integrals and predict infinite dissipation. The standard way out is
to invoke a slip length. We have developed a method based on monitoring the
motion of a drop using a split diode detector to measure the mechanical Q of a
liquid drop on a plate. We are investigating the effect of viscosity, contact
angle, and vibration amplitude on the Q graph. Picture.
- Nucleation in cesiated containers
top
- Wetting affects nucleation. If a phase wets a wall, there is no barrier to
nucleation, and a thin film continuously grows as coexistance is approached. If
the nucleating phase does not wet the wall, there is a finite barrier to
nucleation, and supercooling is possible. Petterson and Saam pointed out the
possibility of supercooling phases of liquid helium using containers with cesiated
surfaces. We have devloped methods for making containers with cesiated walls and
have investigated the supercooling of helium and argon using a calorimetric
technique. We find no supercooling, so despite our best efforts, there are
apparently microscopic nucelation sites. This may be due to the fact that we have
been unable to find a material that cesium wets completely. In the course of
these experiments, however, we discovered a completely different route to
supercooling.
- Superfluid Fog: Nucleation of supersaturated vapor in an evaporating liquid
top
- A liquid coexisting with its vapor in a container with a temperature gradient will
transfer heat and mass from the hot end to the cold end via evaporation and
condensation. A careful analysis of this non-equilibrium process leads to some
surprising and non-intuitive predictions: very larger temperature jumps develop
near the liquid-vapor interfaces, and it is possible for the temperature in the
vapor to be inverted so that the temperature near the hot evaporating fluid is
actually colder than it is near the cold end where the fluid condenses. This
effect is a clear prediction of non-equilibrium thermodynamics and has been seen
in computer simulations but has never been observed in conventional fluids due
to the difficulties in making the measurements. In the liquid helium system, the
effects are so strong that with a modest evaporative flux, the gas in the interior
can be driven into a supercooled metastable state. When the supercooling becomes
sufficiently strong, the vapor undergoes homogeneous nucleation and forms a dense
fog, which we have recently documented with video microscopy in our optical dewar.
Supercooling Helium Vapor: Nucleation and Fog
Formation induced by Strong
Evaporation
 See the video See the video
|
|
|
|
|
| All material on this site is the intellectual property of the Taborek/Rutledge research group. |
|
